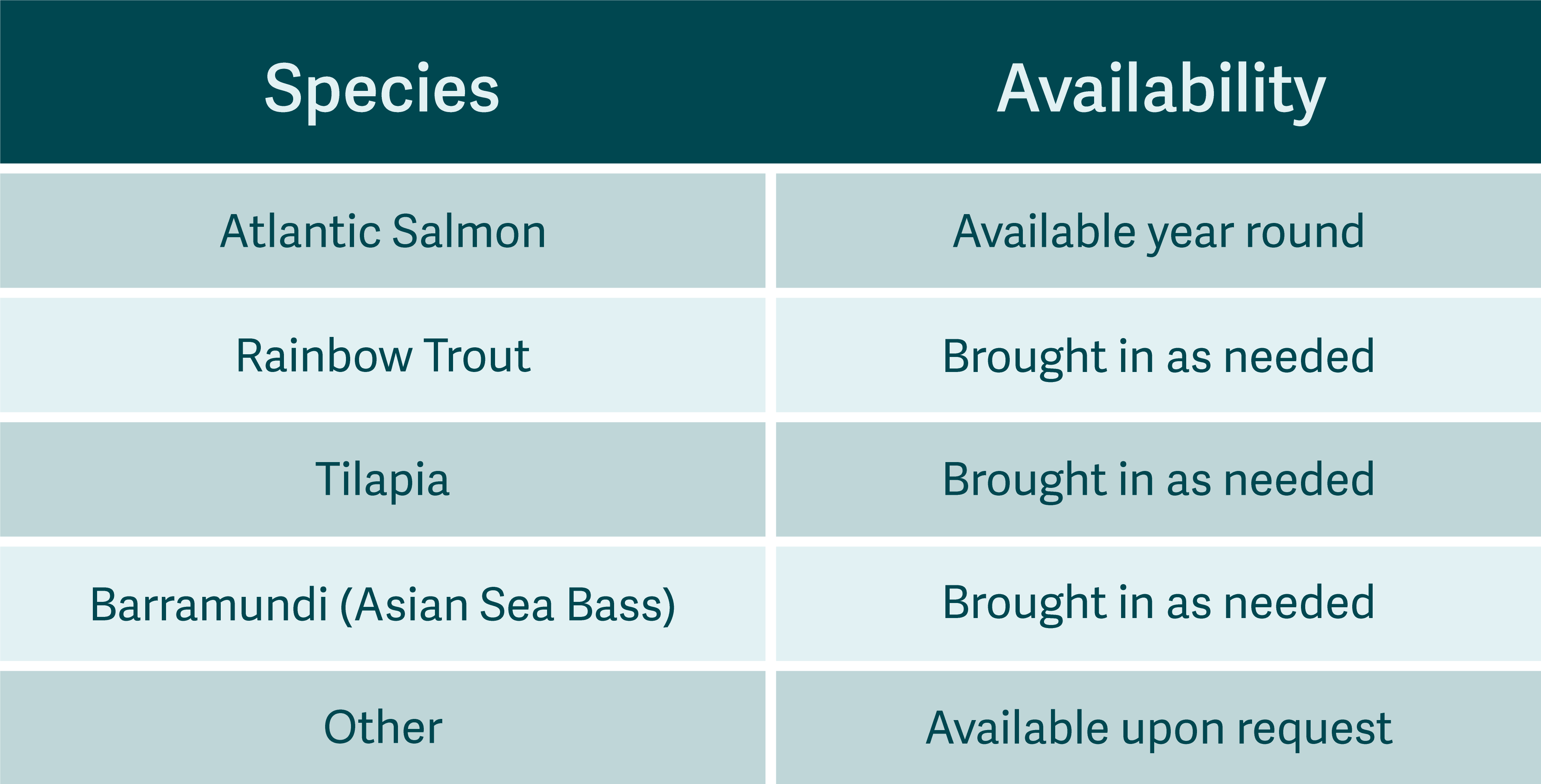
Bacterial Confirmatory Loss Testing
Onda maintain compliance to the EudraLex GMP Guidelines as these pertain to Quality Control
Testing.
GMP is designed to ensure products are consistently produced and controlled to meet the quality and safety standards required for their intended use.

CFIA & USDA Quality Control Testing piggybacks off the GMPs, utilizing the same programs that mitigate the risks associated with final market release while maintaining compliance to the Health of Animals Act and the 9CFR.
Onda molecular testing equipment and assays maintain compliance to the GMP computer system Annex 11 and assay validation requirements.


The client owns the testing methodologies, and our Quality Control team validates them in accordance with the specific requirements of the client.
The Quality Control laboratory uses challenge seed banks that are under the ownership of the client. Onda holds approvals for the utilization of a wide range of aquatic and zoonotic pathogens, encompassing bacteria, intracellular bacteria, and viruses.
Challenge banks are housed in an alarmed ultra low freezer that have undergone GMP qualification.
All effluent water and waste are thoroughly decontaminated prior to discharge.
Onda also specialize in providing comprehensive support for the development of both in vivo and in vitro methods.
EU GMP Certification - Lower Saxony State Office for Consumer Protection and Food Safety (LAVES)
Client Specific as Needed
CFIA Veterinary Biologics Facility License
Compliance to the Health of Animals Act
CFIA AQC1 Containment Standard
CFIA AQC2 Containment Standard
CFIA AQC3 Containment Standard
PHAC CL2 Containment Standard
CCAC Good Animal Practice Certification
USDA-APHIS compliant
Facility Licensing is Client Specific